Key Terms
o Electric force
o Electric charge
o Positive (electric) charge
o Negative (electric) charge
o Atoms
o Protons
o Neutrons
o Electrons
o Conductor
o Insulator
o Semiconductor
Objectives
o Identify the electric force and its similarities to gravity
o Differentiate between positive and negative charge
o Understand a simple model of atoms, including their constituent protons, neutrons, and electrons
o Recognize the essential characteristics of conductors and insulators
Electronics (electrical circuits) are all around us: our computers and cell phones are more-complicated examples, but even simpler things like stoves, lamps, speakers, and digital watches involve the same electrical phenomena. If you've ever opened up a computer or other device, you may have seen a nearly inscrutable maze of wires, components, and electronic chips. But these complicated devices are designed using basic (and in some cases simple) physical principles.
Charge and the Electric Force
Pick up a pen or pencil (or some other light object), hold it above the floor, and release it. That object then falls. You're probably familiar with the terms gravity and gravitational force. The Earth, because of its mass, exerts an attractive force on other objects that pulls those objects toward it. We can see very clearly the way gravity works: it is a force we feel directly (it "weighs us down"), and we see the effects of it on objects in everyday life.
A force that is fundamentally different but similar in many of its characteristics is the electric force. Just as the gravitational force acts on two (or more) masses, the electric force acts on two (or more) electric charges. Electric charges come in two kinds, called positive charge and negative charge. Positive charges are typically identified with the plus sign (+), and negative charges are typically identified with the negative sign (-). Unlike masses, which always exert an attractive gravitational force on each other, charges can exert attractive or repulsive electrical force, depending on which types of charge are involved. Specifically, like charges repel one another, and unlike charges attract one another. Objects without any charge, however, neither experience nor exert an electric force. The illustration below shows the forces involved with two masses (m) and two charges (+ and/or -).
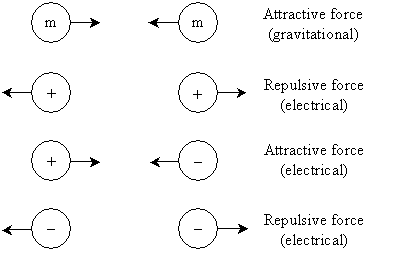
Although we regularly see masses experiencing the attractive gravitational force (for instance, when you release an object and watch it fall), directly observable effects resulting from the electric force a little harder to find. But consider when you rub a balloon on your hair and then "stick" it to a wall--this is an electrical effect. The rubbing action causes a buildup of negative charge that creates an attractive force with positive charge in the wall, causing the balloon to stick, even to the point that it overcomes the gravitational force (i.e., it doesn't fall). A similar electrical effect occurs when you approach a CRT television screen, causing your hair to be "pulled" toward the screen. And you've probably also seen one of the more stunning examples of the electric force: lightning, which is the result of a buildup of charge between clouds or between clouds and the ground.
Atomic Model
According to the reigning physical theory, the material world around us is composed of atoms --small particles of matter that give substances the various properties that we experience (hardness, color, and so on). This theory describes these atoms as central, positively charged nuclei (composed of positively charged protons and uncharged neutrons ) surrounded by negatively charged electrons which "orbit" the nuclei, as illustrated in the simplistic model below. The electric force "pulls" the electrons toward the nucleus in a manner that is in some sense similar to the way the earth pulls an orbiting satellite toward it. (It's not really this simple, but this basic explanation suffices for the purposes of this course.)
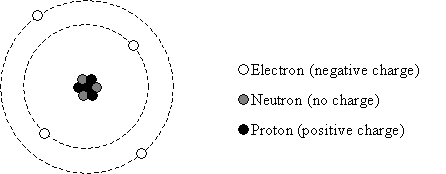
The objects and materials we experience everyday are composed of different types of atoms (defined by the number of protons, neutrons, and electrons that compose them), and atoms are generally neutral, electrically. (That is, the positive and negative charges in the atom are equal in magnitude, causing the whole to seem in many ways like it is without any charge at all--in other words, the positive and negative charges "cancel" one another). Electronics (which we will interchangeably call electrical circuits) use the electric force and the properties of different materials (composed of various atoms) to perform a variety of functions: creating light, displaying the time, performing complex calculations, displaying an image on a screen, and so on.
Conductors and Insulators
For certain atoms, the electrons that surround the nucleus are "loosely bound" and have relative freedom to move from one atom to another. Materials composed of such atoms are called conductors because they allow electric charge to be moved ("conducted") through the material when an electric force is applied. An example of a conductor is a metal such as copper (which is widely used in electrical circuits for its highly conductive characteristics).
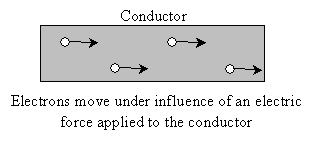
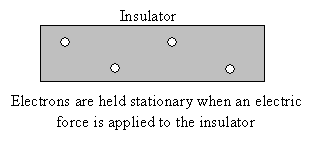
Between conductors and insulators is the group of materials called semiconductors. Semiconductors (such as silicon) are the heart of modern electronics. We will discuss semiconductors in slightly more detail later in the course.
Focus of This Course
Our study of electrical circuits is essentially the study of how an arrangement of conductors and insulators (and, sometimes, semiconductors) behaves when an electric force is applied. The remainder of the course will apply these basic concepts that we discussed above, focusing on sources of the electric force, the function of various components in electric circuits, and how to quantify and measure the characteristics of circuits. We will focus mostly on concepts rather than numbers, although some discussion of mathematics is necessary.
To aid our discussion, we will relate electricity to gravity, thereby giving you a more readily understandable point of reference (or metaphor) to help you grasp the concepts of electronics. The subject of electronics can be daunting and, at times, difficult to understand.
Practice Problem: An electron (white) and a proton (black) are placed as shown below. What will happen, if anything, when the two particles are released?

Solution: In our discussion of the atom, we noted that electrons are negatively charged and protons are positively charged. Because unlike charges exert an attractive electric force (i.e., "opposites attract"), they will accelerate toward each other when released.

Practice Problem: Three materials are arranged as shown below. They are surrounded by air. Will electrons be able to travel from point A to point B? Explain why or why not.
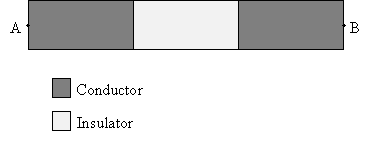
Solution: Conductors allow electrons (negative charge) to flow through them, whereas insulators do not. This arrangement of materials is in air; recall that air is an insulator. Thus, the two conducting areas are actually surrounded by another insulating material.
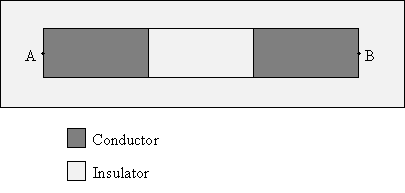
Because there is no continuous path through a conducting material for electrons at point A to reach point B, electrons will not be able to travel to point B from point A.