Key Terms
- Free radical
- Heterolytic cleavage
- Homolytic cleavage
- Photochemical reaction
- Chain reaction
- Initiation step
- Propagation step
- Chain-terminating step
- Polymerization
- Polymer
- Monomer
Objectives
- Recognize the characteristics and electron structure of free radicals
- Explain the creation of free radicals by way of homolytic cleavage
- Describe reaction mechanisms for chlorination of methane and for polymerization using free radicals
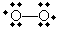
Interestingly, perhaps the most recognizable free radical is diatomic oxygen (O2), which has the following form-note that oxygen in this form does not form a double covalent bond, meaning it doesn't obey the octet rule. Oxygen is, however, a neutral molecule, and each atom has a formal charge of zero.
Although oxygen is a relatively stable free radical, many radicals are highly reactive. Radicals have an important role in many reactions, a few of which we will discuss here, including the formation of polymers (polymerization).
Characteristics and Formation of Free Radicals
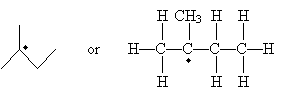
Carbon atoms with an unpaired electron are more stable the more highly substituted they are. Thus, a methyl radical (�CH3) is less stable than a monosubstituted carbon radical (a primary radical), which is less stable than secondary and tertiary radicals. These forms are shown below in order of increasing stability when going from left to right. (The R groups are substituents other than hydrogen.)
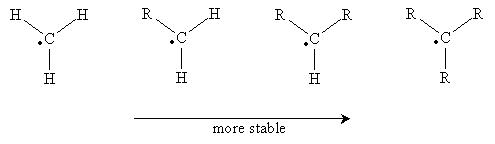
Note that the unpaired electron is not involved in bonding, so it exists in an unhybridized 2p orbital. The remaining orbitals are sp2 hybridized, so they form a trigonal planar arrangement. With increasing substitution, however, the molecule begins to resemble a trigonal pyramidal shape (tetrahedral less one substituent), although it remains closer to the planar form.
When two atoms or groups bonded covalently dissociated in such a way that one atom or group retained both electrons in the bonding pair--this is called heterolytic cleavage. But in some instances, each group retains a single electron, thereby forming two free radicals (assuming the original molecule had an even number of electrons. This type of dissociation is called homolytic cleavage, and it is illustrated below for the specific example of a chlorine molecule. Note the use of a single-barbed arrow (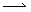
 ) to represent the movement of a
) to represent the movement of a
single electron rather than an electron pair (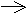 ).
).

Homolytic cleavage can be induced through high temperatures (400�C to 440�C) or by illuminating the chemicals with electromagnetic radiation (light-often in the visible or ultraviolet regions of the spectrum). Use of light is often represented using the symbols h?, which is the product of Planck's constant, h, and the frequency of the radiation, ?. This product is the energy of a photon for this frequency (or wavelength) of light. Photochemical reactions are reactions that involve absorption of light to initiate some step or steps.
Practice Problem: Assume the molecule 2-methylbutane undergoes homolytic cleavage to produce two free radicals: a hydrogen atom and an alkyl radical. Which carbon is most likely to have the unpaired electron?
Solution: This problem requires that we assess relative stability, as the more stable radical form will have a lower energy. The molecule 2-methylbutane is shown below.
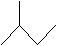
An alkyl radical is more stable when the carbon with the unpaired electron is more highly substituted. The molecule above has one tertiary (trisubstituted) carbon-the carbon at position 2. This carbon is the most likely candidate for homolytic cleavage because the radical form of the molecule is most stable when the unpaired electron is located there.
Free Radical Reactions
An illustrative example of a free radical reaction is the chlorination of methane. This reaction is called a chain reaction because, as we will see, homolytic cleavage of chlorine (the so-called initiation step of the reaction) yields free radicals that can yield a disproportionate amount of the reaction's products.
Following the cleavage step above for chlorine-whether performed at high temperature or in the presence of light-the chlorine free radical attacks methane as shown below.
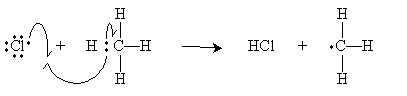
The methyl radical then attacks a diatomic chlorine molecule to form methyl chloride (chloromethane) and a new chlorine radical. Note that these two steps-which constitute the so-called propagation steps of the reaction-do not change the concentration of chlorine radicals. Hence, the methane can be chlorinated indefinitely (theoretically).
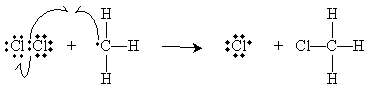
The reaction process is slowed by the less likely process of chain termination (chain-terminating steps ). This occurs whenever two radicals combine to form a molecule with no unpaired electrons, but these reactions are much less likely than the steps shown above. Chain-terminating reactions possible in the case of the mechanism above include diatomic chlorine (via recombination of two chlorine radicals), methyl chloride (via radical combinations rather than radical attack on diatomic chlorine), and ethane (via combination of two methyl radicals).
At room temperature (and above), methane and chlorine are in gas form, and the chlorination process shown above also yields higher-substituted products (CH2Cl2, CHCl3, and CCl4).
Polymerization
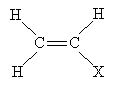
Another important reaction that involves free radicals is polymerization, or the formation of polymers. A polymer (from poly- meaning "many" and -mer meaning "part") is a chain of repeating chemical structures; polymers can be formed by way of radical reactions involving alkenes. One type of polymer structure is shown below, where X is a substituent group. The n indicates that the portion in parentheses repeats n times (typically a large but indefinite number).
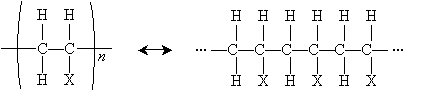
To form a polymer of this form, the following alkene is required. The basic individual unit for each polymer is called a monomer.
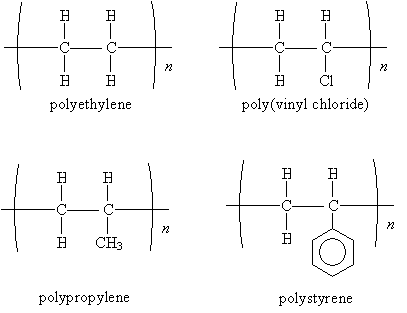
Some common polymers are shown below. Note that the name of the polymer is simply the name of the monomer preceded by the prefix poly. (Often, the common name, rather than the IUPAC systematic name, is used.)
Polymerization can take place by way of a free radical mechanism. Consider the case of a well-known polymer, Teflon. Teflon is identical to polyethylene except that the hydrogen atoms are replaced by fluorine atoms.
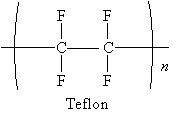
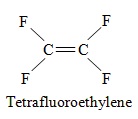
Teflon starts from the alkene tetrafluoroethylene.
To initiate the reaction, tetrafluoroethylene is added to peroxides at 80�C and high pressure (40 to 100 atmospheres). A peroxide has the structure shown below, where R represents a substituent carbon compound (a simple alkane, for instance). Note that these two R compounds need not be identical.
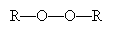
The perioxides cleave homolytically under the above-mentioned conditions to yield two free radicals.
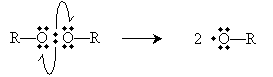
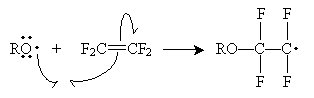
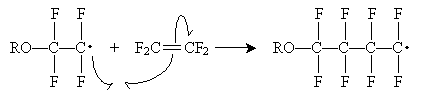
The second step can continue indefinitely to create a polymer of arbitrary length. This same mechanism applies to other molecule chains formed by free radical polymerization.
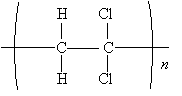
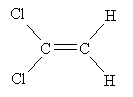
Practice Problem: The molecule 1,1-dichloroethene polymerizes to form a clear wrap material for packaging. Show the mechanism for this polymerization process in the presence of peroxides at 80�C and 40-100 atmospheres.
Solution: The sketch below shows 1,1-dichloroethene.
The peroxides (ROOR), under the conditions described in the problem, cleave homolytically to form two alkoxyl radicals.
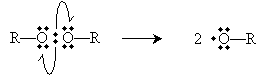
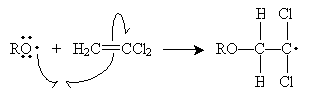
The alkoxyl radical attacks the double bond of 1,1-dichloroethene to form a new radical. Because the carbon at position 1 (to which the chlorine atoms are attached) is more highly substituted than the carbon at position 2, it is more stable when it has an unpaired electron. Thus, the propagation steps are the following.
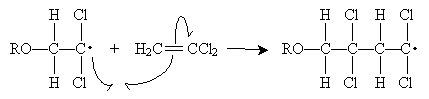
This process continues to form the polymer chain shown below.