Conjugated dienes illustrate the fact that combinations of functional groups can yield molecules that behave differently from molecules with just the individual functional groups.
Key Terms
- Isolated diene
- Cumulated diene
- Conjugated diene
- 1,2-addition
- 1,4-addition
- Dienophile
Objectives
- Differentiate between different types of dienes
- Explore how dienes behave differently from alkenes for addition of a hydrogen halide
- Describe the mechanism for the Diels-Alder reaction
Combinations of substituent groups can yield chemical reactivity different from that of substances containing a single group. In this article, we will investigate one such case: conjugated dienes.
As the name indicates, a diene is a molecule containing two double bonds between carbon atoms. Dienes can be one of three types. The first is an isolated diene, in which the two double bonds do not involve adjacent carbon atoms. An example is shown below.

Another type is a cumulated diene. In this case, a single carbon participates in two double bonds simultaneously, as with the example below.
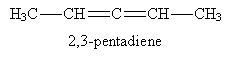
Finally, dienes can also be conjugated, as shown below. In a conjugated diene, the double bonds are separated in the carbon chain by a single bond.

We will focus on conjugated dienes and their chemical behavior.
Practice Problem: What type of diene is the molecule below?
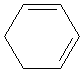
Solution: The molecule shown above, 1,3-cyclohexadiene, is a conjugated diene, because the two double bonds are separated by a single bond. Although it may appear to be an isolated diene from one perspective, it has the characteristics of a conjugated diene and therefore can behave as such.
As it turns out, conjugated dienes are the most stable of the three types. Recall from our discussion of benzene that the unhybridized 2p orbitals in the carbon atoms overlap to allow the further delocalization of electrons. And, as we have discussed, delocalization of electrons increases stability, as in resonance forms.
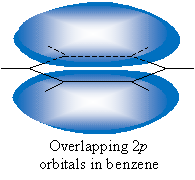
The same effect, although it is less pronounced, takes place in the case of conjugated dienes. Four successive carbon atoms in a chain have unhybridized 2p orbitals that overlap in a similar manner, as illustrated below. This type of overlap does not occur in the case of isolated and cumulated dienes.

Furthermore, conjugated dienes can have one of two stereo configurations: s-cis and s-trans. Note that the special notation (rather than just cis- or trans- is used because the "center" of the configuration is the single bond between the two vinyl groups, rather than a double bond.

Reactions of Conjugated Dienes
We consider two important reactions involving dienes: addition of hydrogen halides and the Diels-Alder reaction.
Hydrogen halides add to dienes in a manner similar to the way they add to alkenes, but the characteristics of dienes-particularly the overlap of 2p orbitals of the carbon atoms, affect the way the addition takes place. Consider the mechanism for the addition of hydrogen bromide to 1,3-cyclohexadiene. The overall reaction is given below.
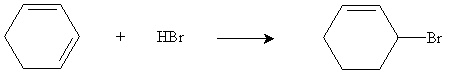
To start, hydrogen bromide acts as an electrophile and reacts with one of the double bonds in the cyclohexadiene.
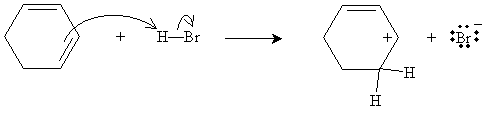
Note the location of the proton when it is added to the cyclohexadiene: this placement is because when the positively charged carbon atom is adjacent to the double-bonded carbons, the unhybridized 2p orbitals can still overly, allowing for greater electron delocalization. Thus, this form is lower in energy-and hence more stable-than the alternative.
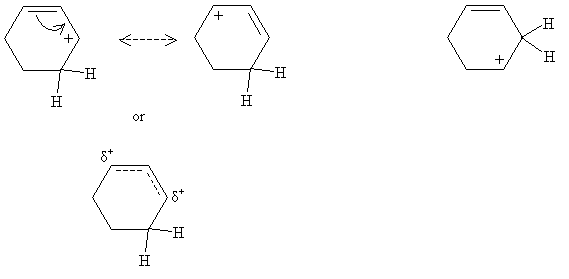
This overlapping of unhybridized 2p orbitals is shown by way of the resonance structures for the form on the left. This is the preferred form. The form on the right does not have resonance and is therefore higher in energy (the carbocation is less stable). The next step in the mechanism involves attack of the relatively nucleophilic bromide ion on the carbocation. Note that in this case, it doesn't matter which carbocation form is attacked-the product is the same in both cases (3-bromocyclohexene).
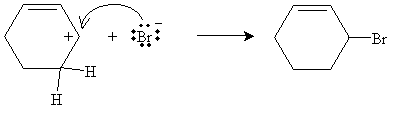
But the addition of the halide ion may yield two different products for certain molecules. Consider 1,3-butadiene; the two carbocation resonance forms below are possible, but the more highly substituted form (on the left) is more stable and, therefore, more likely to react with the bromide ion.

Two different products of addition of hydrogen bromide result in this case: 3-bromo-1-butene (left) and 1-bromo-2-butene (right).

Because of the stability of its corresponding carbocation, 3-bromo-1-butene is the dominant product of this reaction at very low temperatures. Interestingly, however, higher temperatures (room temperature, for instance) cause the product on the right to be more abundant. This is because the two products can interconvert at sufficiently high temperatures, as illustrated below. The product on the right dominates at higher temperatures because it is the more stable of the two.
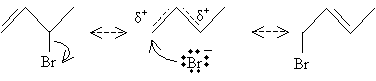
The addition of hydrogen bromide to 1,3-butadiene is classified as 1,2-addition (in the case of 3-bromo-1-butene), as the proton adds to carbon 1 and bromide ion adds to carbon 2 in the chain of the original molecule (note that the carbon numbering reverses between the initial molecule and the product molecule-this is simply a quirk of nomenclature). In the case of 1-bromo-2-butene, 1,4-addition occurs, as the proton adds to carbon 4 and the bromide ion adds to carbon 1.
A unique reaction is the Diels-Alder reaction, which involves addition of an alkene and a conjugated diene to form a cyclohexene ring. Consider the simple case of 1,3-butadiene and ethene (ethylene). Note that this reaction requires 1,3-butadiene to have the s-cis conformation. The unhybridized 2p orbitals of the carbon atoms sharing double bonds overlap, and the ? electrons rearrange to form the ring structure in a single step, as illustrated below.
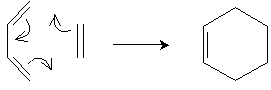
Ethylene, which reacts with the diene, is called a dienophile ("diene seeker").
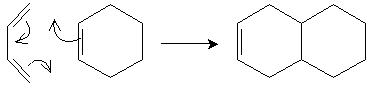
Practice Problem: Draw a diagram of the product of a reaction between 1,3-butadiene and cyclohexene.
Solution: This reaction follows the pattern of the Diels-Alder reaction discussed above, but the result is a polycyclic molecule. Since we have not discussed nomenclature for these types of molecules, it suffices to draw the resulting structure.