Key Terms
- Aliphatic hydrocarbon
- Alkane
- Methane
- Ethane
- Propane
- Butane
- n-butane
- Isobutane
- International Union of Pure and Applied Chemistry (IUPAC)
- Cycloalkane
Objectives
- Distinguish between simple alkanes and cycloalkanes
- Use the IUPAC rules of nomenclature to systematically name alkanes and cycloalkanes
Alkanes
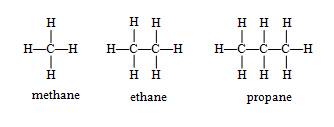
The simplest alkane is methane, which has a molecular formula CH4. Ethane contains two carbon atoms and has a molecular formula C2H6, propane has a molecular formula C3H8, and butane has a molecular formula C4H10. Note that methane, ethane, and propane each have only one isomer:
Butane, on the other hand, has more than one isomer (as do alkanes with more than four atoms).
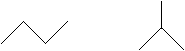
The molecule on the left is called n-butane, where the 'n' stands for "normal." Note that in this case, the molecule is an unbranched chain of carbon atoms, unlike the isomer on the right. All unbranched alkanes of four or more carbon atoms are preceded by "n-" to identify them as such. The isomer on the right is called isobutane.
IUPAC Nomenclature
The rules for organic nomenclature are specified by the International Union of Pure and Applied Chemistry (IUPAC). Beyond butane, alkanes are named in a manner similar to polygons (e.g., pentagon, hexagon, and so on). The n-alkanes with up to 10 carbon atoms are shown below along with their general name (omitting the n-), except for methane, ethane, and propane.
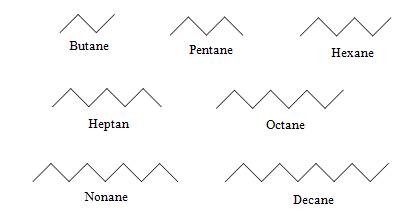
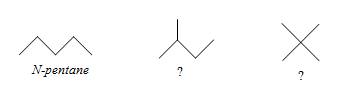
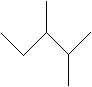
If you look at the molecular formula for each of these alkanes, you will notice that as the number of carbon atoms increases, so does the number of possible isomers. Thus, although the first three alkanes (methane, ethane, and propane) are sufficiently descriptive names, an unqualified name like pentane for a molecule with five carbon atoms is ambiguous. As we noted above, n-pentane is the isomer shown with the other n-alkanes above, but how do we identify different isomers with the same molecular formula, such as those shown below?
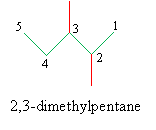
This brings us to the IUPAC rules for naming alkanes. By following a simple, systematic process, you we can easily find an unambiguous name for a given alkane. The rules are summarized below, and we will use the following example molecule to illustrate the procedure.
1 .Identify the longest continuous chain of carbon atoms in the molecule. The molecule is broadly identified by the IUPAC name for this chain.
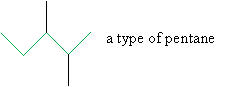
2. Identify the groups attached to the chain identified in step 1. (Simple unbranched alkane chains whose point of attachment is at either end of the chain are named by removing the -ane suffix and replacing it with -yl.)

3 .Number the carbons in the chain identified in step 1-from one to n, where n is the number of carbons in the chain-such that the first branch group is at the carbon with the smallest number.
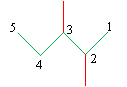
4. Assign the correct IUPAC name, listing the attached groups in alphabetical order and ending with the name of the chain from step 1.
Remark 1. If more than one possibility presents itself in step 1, choose the chain that has the largest number of substituent groups.
Remark 2. If both options for numbering the chain in step 3 are acceptable, choose the one that assigns the smallest number to the group that is first alphabetically.
Remark 3. Multiple instances of the same substituent group should be named using the prefixes di-, tri-, tetra-, and so on.
Let's look at another example.
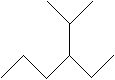
We have two different options for choosing the longest continuous chain (step 1):

But the option on the right contains more substituent groups, so we use that option (remark 1). Next, we number the chain according to step 2.
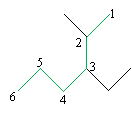
This molecule is a variant of hexane. Next, we identify the substituent groups (step 3) and then write the IUPAC name for the molecule (step 4).
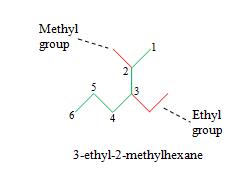
Pay careful attention to the way the name is written: hyphens are used between the numbers identifying the locations of each group, and the name of the base alkane is attached-without a space-to the end of the name. If the same alkyl group appears more than once, the group name appears once but is preceded by a list of location numbers (in ascending order), separated by commas (as in the example we used when presenting the procedure).
Practice Problem: Determine the IUPAC name for the following molecule.
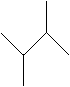
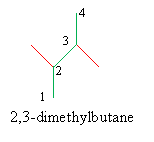
Solution: Follow the rules given above. First, find the longest chain (the base molecule-butane, in this case), then number the carbons in that chain. Note that both numbering schemes are correct owing to the molecule's symmetry. Next, identify the alkyl groups (methyl groups) and write the IUPAC name.
Practice Problem: Determine the IUPAC name for the following molecule.
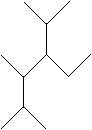
Solution: Again, use the IUPAC rules. The longest chain in this case is a hexane. We must choose the hexane chain so that the number of substituent groups is maximized. Although several hexane chains are equally correct, at least one is not.
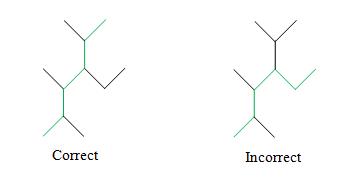
Number the hexane chain, identify the alkyl groups (three methyl groups and one ethyl group), and write the IUPAC name. Note that we must number the chain such that the ethyl group is at position 3, not position 4 (see remark 2 in the rules above).
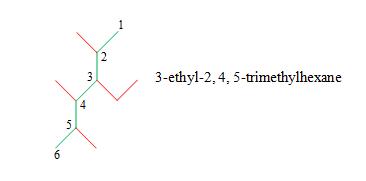
Cycloalkanes
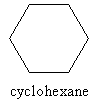
We've now seen a number of alkanes, both branched and unbranched. As long as these alkanes contain no "loops" (or rings), the molecular formula is always CnH2n+2. But sometimes, carbon molecules do indeed form rings; if these rings involve only carbon and hydrogen and only single bonds between the carbon atoms, the molecules are called cycloalkanes. An example of a cycloalkane is shown below.
As with alkanes, IUPAC rules specify a systematic nomenclature for naming cycloalkanes. The system is similar to that of alkanes but with a few subtleties. First, note that cycloalkanes can also appear as substituent groups as well as the "main" molecule: in such cases, we make the same substitution of the -yl suffix for the -ane suffix. For instance, if cyclohexane appears as a substituent group in a molecule, it is called a cyclohexyl group. The procedure for naming cycloalkanes is given below, along with an example molecule.
1. Identify the IUPAC name for the cycloalkane according to the number of carbon atoms in the ring.
2. If substituent groups are attached to more than one carbon atom in the ring, number the atoms in the ring such that the group that is first alphabetically is at position 1 and the next group is at the lowest-number position.
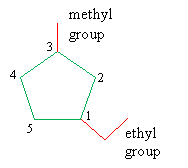
3. Assign the correct IUPAC name, again listing the groups in alphabetical order and ending with the name of the cycloalkane.
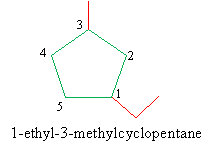
Remark 1. If one or two groups are attached to only one carbon atom in the ring, no numbering is necessary: the location of these groups is necessarily 1, so it need not be stated explicitly.
Remark 2. If the molecule contains a chain (not including any part of the ring) that has more carbon atoms than the ring, then the molecule should be named using the rules for alkanes, with the ring being a cycloalkyl group.
Practice Problem: Determine the IUPAC name for the following molecule.
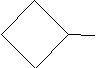
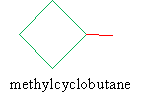
Solution: Clearly, this molecule is a form of cyclobutane. Because it includes only one substituent alkyl group, we need not number the carbons in the ring. The IUPAC name is thus simple.
Practice Problem: Determine the IUPAC name for the following molecule.
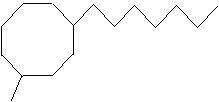
Solution: Because the longest substituent alkyl group connected to the ring is a heptyl group (seven carbons), the base molecule is cyclooctane. Number the ring such that the heptyl group is at position 1 (since it comes before "methyl" alphabetically). (Only the first few numbers are shown in the diagram.) Write the IUPAC name.
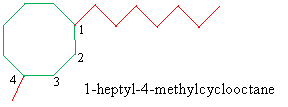
These rules for naming alkanes and cycloalkanes are critical to the study of organic chemistry. These rules server as a foundation for naming more-complicated molecules that include other elements in addition to carbon and hydrogen.