Key Terms
- Enolate
- Enol
- Tautomer
- Enolization
- ? halogenation
- Aldol condensation
Objectives
- Recognize the relationships between enols, enolates, and aldehydes and ketones
- Explain the acidity of ? hydrogens in light of resonance and dipole moments in carbonyl groups
- Analyze mechanisms for ? halogenation and aldol condensation reactions
Ketones and aldehydes are two very important types of organic compounds. This article will focus on enols and enolates, which are two closely related forms that give ketones and aldehydes many of their reactive properties. The carbonyl group is dipolar because the oxygen, being more electronegative than the carbon, tends to draw the electrons in the molecule (particularly from nearby atoms) toward itself, leaving a net charge imbalance.
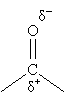
To some extent, this shift in the molecule's electron probability density (the square of the wave function) affects the substituents of the carbonyl group. Those closest to the carbonyl group are affected the most, and the effect diminishes farther from the carbonyl group. For easy reference to relative location from the carbonyl group, associated atoms are identified using Greek letters ?, ?, ?, and so on. Thus, in 4-heptanone, the carbon atoms at positions 3 and 5 are ? carbons, those at positions 2 and 6 are ? carbons, and those at positions 1 and 7 are ? carbons. (The hydrogen atoms attached to these carbons are also identified by the same symbol assigned to the corresponding carbon atom.)
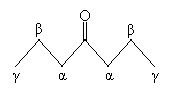
This nomenclature can proceed indefinitely to the end of the carbon chain, but we will focus mainly on atoms in the ? position.
Enols and Enolates
Because the oxygen in the carbonyl group draws electron density away from the rest of the molecule, the hydrogen atoms bonded to the ? carbons (in particular) become more positively charged, which increases their ability to dissociate from the molecule (in other words, these hydrogens are more acidic). Furthermore, note that when an ? hydrogen is dissociated, two resonance forms of the resulting ion are possible. (For illustration purposes, 3-pentanone is used.)
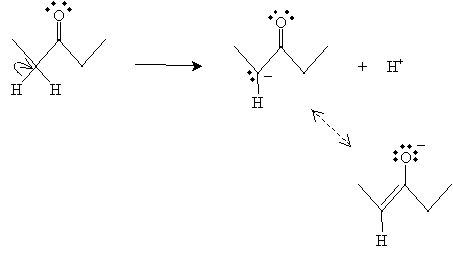
The lower resonance form, which is called an enolate ion (or enolate), is the more stable of the two forms and, therefore, more prevalent. The resonance forms stabilize the enolate, further increasing the acidity of the ? hydrogen. Note that the enolate ion in the example is similar to the neutral molecule below.
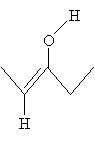
This structure, which is an alkene with a hydroxyl group attached to one of the doubly bonded carbon atoms, is (not surprisingly) called an enol. Enols and certain corresponding aldehydes or ketones are tautomers, which are forms that differ only by way of a movement of an atom or functional group. Note that the enol form of 3-pentanone is formed by moving the hydrogen atom from the ? carbon to the oxygen atom in the carbonyl group.
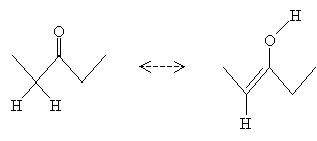
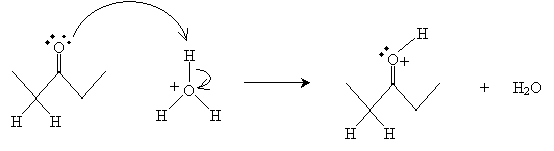
The process of converting a ketone or aldehyde to an enol is called enolization. This process can take place by either an acid-catalyzed or base-catalyzed mechanism. In the acid-catalyzed case, the hydronium ion donates a proton to the oxygen atom, forming water and a cation.
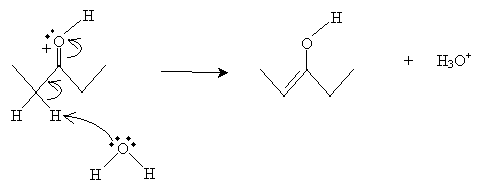
In the next and final step, a water molecule captures an ? proton to form the enol.
Practice Problem: What is the enolate form of cyclohexanecarbaldehyde?
Solution: Cyclohexanecarbaldehyde is shown below.
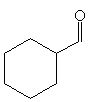
The corresponding enolate ion is formed by dissociating a hydrogen atom from the ? carbon atom and then rearranging the electron "locations" slightly.

Solution: Acetone is shown below. The enol form is obtained by transferring an ? hydrogen to the oxygen atom and rearranging the bonds appropriately.
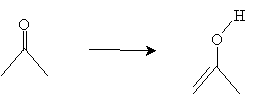
The structure on the right is the enol tautomer of acetone.
Practice Problem: Propose a mechanism for base-catalyzed enolization of 3-pentanone.
Solution: The ? protons of ketones and aldehydes are acidic. Thus, a base will tend to interact with one of these protons as follows.
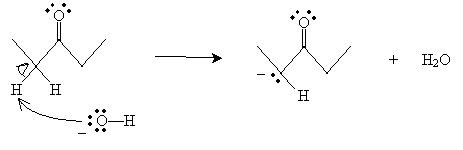
But the product in this case has a resonance form: an enolate ion.
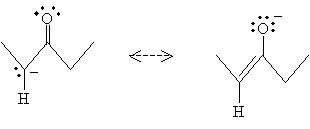
Water can then interact with the oxygen atom as follows to form the enol.
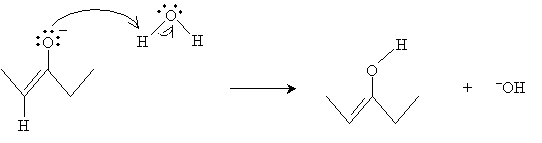
Reactions Involving Enols and Enolates
Although we cannot survey all the many reactions that aldehydes and ketones undergo by way of their enol and enolate forms, we can review two that will provide some insight into the behavior of these compounds.
One such reaction is ? halogenation. In this case, an acidic solution containing a halogen such as chlorine will substitute a halogen atom for an ? hydrogen in a ketone or aldehyde. Consider the example of acetone, for instance. The first step of the reaction involves formation of the enol of acetone; this process is identical to the mechanism of acid-catalyzed enolization discussed above. The result is shown below.
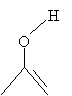
Chlorine then acts as an electrophile and attacks the double bond as shown below.

Finally, a water molecule "steals" a proton from the oxygen atom, yielding a chlorinated ketone (1-chloroacetone or 1-chloropropanone).

Another representative reaction is the so-called aldol condensation. A condensation reaction combines multiple molecules into one-for instance, two alcohols (such as ethanol) into an ether (diethyl ether). The aldol condensation, converts an aldehyde (or ketone) into a compound with a hydroxyl group and a formyl group (carbonyl group). The reaction takes place in basic conditions (hydroxide ions and water) according to the following overall description for the case of propanal. Note that this the reactant and product are in equilibrium, with the product generally dominating in the case of aldehydes and the reactant dominating in the case of many ketones.
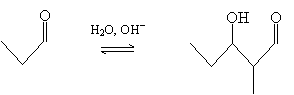
The mechanism for this reaction begins with base-catalyzed formation of the enolate ion for propanal.
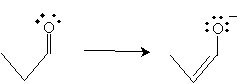
The enolate then acts as a nucleophile, attacking the carbonyl group of the propanal molecule.
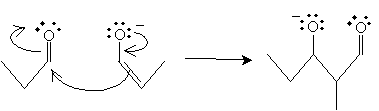
Finally, the resulting anion product reacts with water to yield an aldol and hydroxide ion.
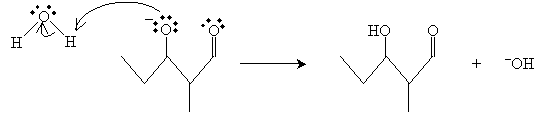
The product in this case is 3-hydroxy-2-methylpentanal.
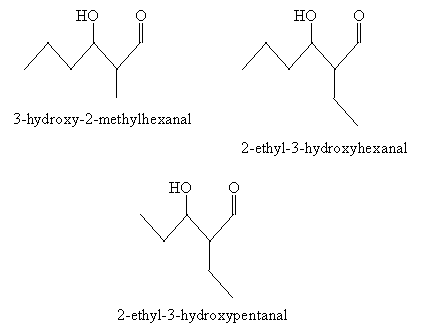
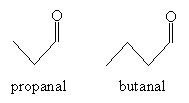
Practice Problem: A mixed aldol condensation results from the introduction of two different aldehydes. What are the possible products of an aldol condensation of propanal and butanal?
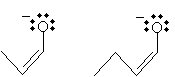
Solution: Propanal and butanal are shown below. Either one of these molecules can form an enolate ion in a base solution.
The corresponding enolates are the following.
Following the pattern of the aldol condensation discussed earlier, several products are possible. For instance, the enolate of propanal and unaltered propanal can combine to form 3-hydroxy-2-methylpentanal.
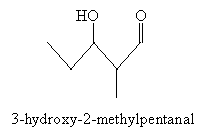
Other potential combinations of enolates (of propanal or butanal) and aldehydes (propanal or butanal) are shown below.