Amines are important chemicals in biological processes, and are relatively basic compounds in the context of organic chemistry.
Key Terms
- Amine
- Azide
- Reduction of an azide
- Reductive amination
- Imine
Objectives
- Identify amines and their basic characteristics
- Name amines according to IUPAC conventions
- Describe reactions involving amines
In this article, we focus particularly on a type of nitrogen-based organic compound: amines. An amine is structurally similar to ammonia (NH3), except that it substitutes one or more of ammonia's hydrogen atoms with an alkyl group. Amines, because they are Lewis bases (or electron donors) are best described as nucleophiles. Amines can contain oxygen, and they are not necessarily primary molecules. As you will see below, they may also have as few as one nitrogen atoms.
Alkyl Substitution of Amines and Amine Nomenclature
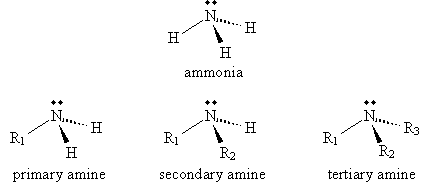
Amines are described by their level of substitution relative to ammonia. Where R1, R2, and R3 are alkyl groups, amines are described as shown below.
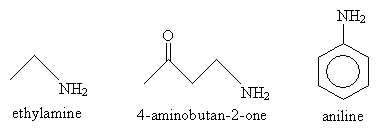
Nomenclature for amines divides into two basic components: one for alkyl substitution of the nitrogen atom and one for aryl substitution. In the former case, the relevant suffix is -amine; molecules where other functional groups are present, the prefix amino- is used. In the latter case, the base molecule is aniline. Some examples are shown below.
When the nitrogen has more than one alkyl substituent, the prefix N- serves to identify the location of these substituent groups. Note that N- serves the same function as numbers in a carbon chain. Thus, for example:
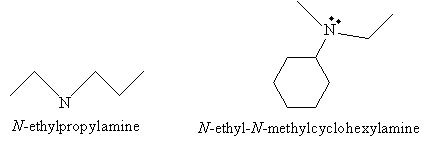
This N- nomenclature applies equally to both alkyl- and aryl-based amines.
Practice Problem: Determine the IUPAC name for the following molecule.
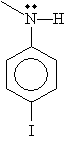
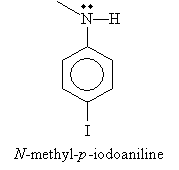
Solution: From the discussion above, we know that this molecule is an aryl-based secondary (disubstituted) amine. The base molecule is analine, and the benzene ring is substituted with an iodide group para to the amine. The nitrogen atom is also bound to a methyl group. Thus, the IUPAC name is N-methyl-p--iodoaniline.
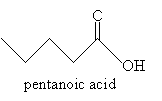
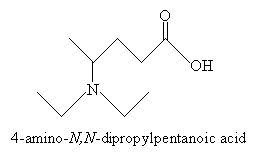
Practice Problem: Sketch the molecule 4-amino-N,N-dipropylpentanoic acid.
Solution: The base molecule in this case is pentanoic acid: start by drawing this portion.
Next, note that the amine group is situated at carbon 4, and the nitrogen is also substituted with two propyl groups. Thus, the molecule in its complete form is shown below. (You may choose to show or omit the nonbonding electron pair of the nitrogen atom. It is included below for completeness.)
Characteristics of Amines
Note that hydrogen bonding can occur when an electronegative element like oxygen forms dipole-dipole weak bonds with hydrogen. Nitrogen, although not as electronegative as oxygen, can also form hydrogen bonds. Owing to these bonds, propylamine has a higher boiling point than butane, but not as high as propanol (hydrogen bonds involving oxygen are stronger than those involving nitrogen).
Owing to the presence of the non-bonding electron pair on the nitrogen atom, amines are weak bases (electron donors, according to the Lewis model). Furthermore, they are important chemicals for biological processes.
Synthesizing Amines
We consider two (relatively) simple reactions for synthesizing amines: reduction of azides and reductive amination. The first reaction involves an azide as a reactant: an azide is shown below.
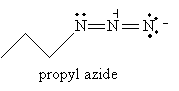
We will not discuss nomenclature for azides in any greater depth, but you can easily gain some insight by way of the example below, along with everything else we have studied thus far regarding IUPAC conventions.
Reduction of an azide can be performed in a number of ways. One is to add a reagent such as lithium aluminum hydride (LiAlH4) in ethoxyethane (diethyl ether) and water. The overall reaction for propyl azide is shown below.
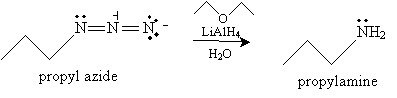
Another, more familiar method of reducing an azide to an amine involves a catalyst, such as platinum, and hydrogen.
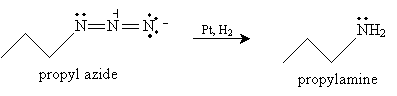
A second synthesis reaction is reductive amination, which converts an aldehyde or ketone to an amine. The overall reaction is shown below for the case of acetone.
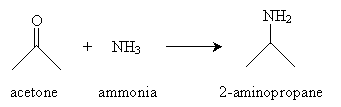
The first step of the reaction involves formation of an imine by way of ammonia and acetone undergoing an acid-base reaction in the presence of water. We continue to use acetone as an example molecule, but the same process applies similarly to other ketones and to aldehydes. The ammonia first acts as a base (nucleophile), attacking the carbon atom in the carbonyl group.
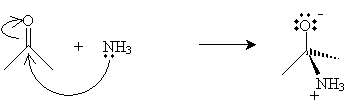
In the next step, water removes a proton from the nitrogen atom (forming a hydronium ion), and a hydronium ion donates a proton to the negatively charged oxygen atom.
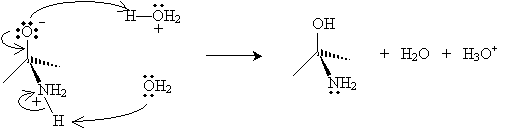
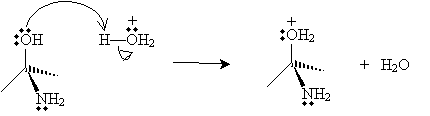
The product in this case then dissociates a water molecule, leaving a new cation with two resonance forms.
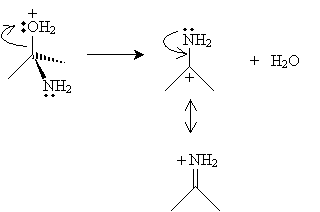
Finally, a water molecule receives a proton from the nitrogen atom, yielding a hydronium ion and an imine.
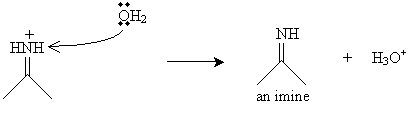
From the imine, we can synthesize an amine by reducing the imine using a catalyst like nickel or platinum in the presence of hydrogen.
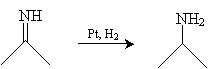
Practice Problem: A chemist wants to synthesize 1-methylpentylamine. How should he proceed if he wants to yield this product by way of reductive amination?
Solution: First, determine the chemical structure of 1-methylpentylamine.
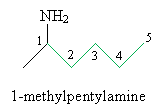
Next, determine which reactants are required to yield 1-methylpentylamine. In this case, 2-hexanone is required, along with ammonia.
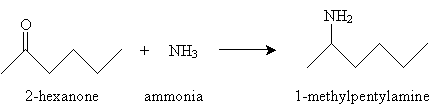
The reactant 2-hexanone interacts with ammonia and water to yield the imine below.
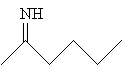
This imine can then be reduced in the presence of hydrogen (H2) and a catalyst, such as platinum, to yield 1-methylpentylamine.
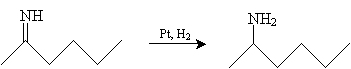
Reactions of Amines
For brevity, we will only consider one type of reaction involving amines. As with other functional groups, numerous other reactions can occur, depending on the conditions. The reaction we will consider here involves alkyl halides and primary amines. Consider, for instance, propylamine and ethyl chloride (ethylene chloride, or chloroethane). The first step of the reaction is a two-molecule nucleophilic substitution (SN2 mechanism).
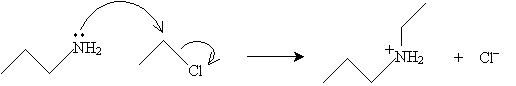
The amine, however, is a weak base, whereas the cationic intermediate above is acidic (an electrophile). Thus, the final step of the reaction is the following.

The product, then, is a secondary amine (in this case, N-ethylpropylamine).
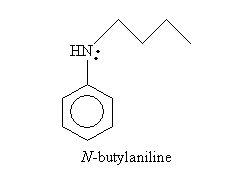
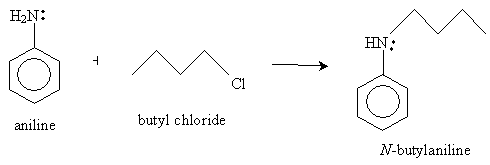
Two possible reactants that can yield this product by way of nucleophilic substitution are aniline and butyl chloride (1-chlorobutane). The overall reaction is shown below; the mechanism is parallel to that discussed earlier.