Introduction
Before taking a closer look at the characteristics and reactions of organic molecules with various functional groups, it helps to review nomenclature for a few more important types of molecules.
Key Terms
- Alkyl halide
- Functional class (nomenclature)
- Substitutive (nomenclature)
- Alcohol
- Hydroxyl (functional group)
- Alkene
- Alkyne
- Cycloalkene
- Cycloalkyne
Objectives
- Recognize molecules containing several common functional groups (alkyl halides, alcohols, alkenes, and alkynes)
- Apply the IUPAC rules of nomenclature to these compounds
Alkyl Halides: Nomenclature
Although hydrogen sits atop lithium in the first column (group) of the periodic table, it is in many ways similar to the halogens (fluorine, chlorine, and so on) in its behavior and characteristics: for instance, it is one electron short of a full valence shell and typically exists as H2, just like the halogens (F2, Cl2, and so on). Thus, carbon compounds that include halogens are, conceptually at least, similar to those that just include hydrogen (alkanes, for example). An alkyl group connected to a halogen is called an alkyl halide, a simple example of which is shown below.
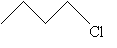
Alkyl groups are often represented using the shorthand 'R' and halogens using the shorthand 'X,' so that the molecule above (a form of butane) would be drawn as follows, where the R represents a butyl group and X represents the chlorine atom in this particular case.
R-X
In general, this shorthand represents any alkyl group R bound to a halogen X.
IUPAC nomenclature for alkyl halides follows the general pattern presented for alkanes, but it diverges slightly into two different but equally systematic approaches. The functional class approach divides the molecule neatly in two: an alkyl group and a halide, which are expressed as two separate words (unlike the procedure we have used thus far). The carbon to which the halogen is bound is designated as position 1, and the longest carbon chain from that position is then used as the base of the alkyl group. Because the halogen is assumed to be at position 1, no number is required in the name (although numbers may be required for substituent groups in the overall alkyl group). All the typical IUPAC rules apply to the alkyl group. Thus, our example molecule above would be named butyl chloride.
Practice Problem: Use functional class nomenclature to identify the molecule below.
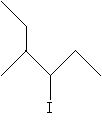
Solution: According to the functional class approach, the carbon to which the halogen is attached is located at position 1. Identify the longest chain from that carbon as the base of the alkyl group and number it accordingly.
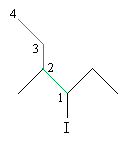
Use the IUPAC rules to name the substituent groups (shown in red below), then write the complete name of the molecule.
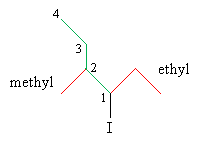
The molecule is thus 1-ethyl-2-methylbutyl iodide.
Although the functional class approach to nomenclature is used by some organic chemists, we will rely mainly on substitutive nomenclature, since it more closely parallels the IUPAC rules for alkanes. In this approach, halides are treated like other functional groups (and alkyl groups) on the longest carbon chain in the molecule. The chain should be numbered, however, so that the halogen is at the lowest-number position; the alphabetical order of alkyl groups is a secondary consideration. The halide functional group is named by replacing -ine in the element name with -o. Thus, our example molecule has a slightly different (but equally descriptive) name in this approach:
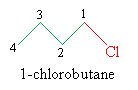
The names 1-chlorobutane and butyl chloride refer to the same molecule, and you should be aware of both naming styles.
Practice Problem: Use the substitutive approach to name the molecule shown below.
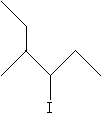
Solution: In this case, we must find the longest carbon chain regardless of the position of the halogen. Our numbering scheme, however, should place the halogen at the lowest-number position.
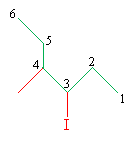
The molecule is thus 3-iodo-4-methylhexane. This same molecule can also be called 1-ethyl-2-methylbutyl iodide.
Alcohols: Nomenclature
Another functional group is the hydroxyl group, or OH, which, when bound to an alkyl group, forms an alcohol. The rules for naming organic molecules with hydroxyl groups are similar to those for naming alkyl halides, except that the hydroxyl functional group takes precedence over halogens when numbering the carbons, just as halogens take precedence over alkyl groups. An alcohol is named by ending the name of the carbon chain with -ol instead of -ane, and it is preceded by a number that identifies the position of the hydroxyl group. This is the substitutive nomenclature; the functional class nomenclature follows that for alkyl halides, but the second word is alcohol rather than a halide name. Thus, for instance, we name the molecule below as follows:
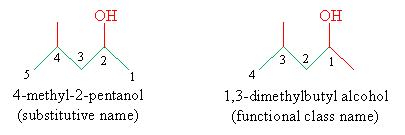
Updated IUPAC rules specify that the substitutive name move the number of the location for the hydroxyl group to just before the -ol suffix, however. Thus, although you may encounter substitutive names for alcohols such as the one given above, you may more likely encounter the name 4-methylpentan-2-ol. This latter approach is often a necessity for complex molecules with many different functional groups.
Practice Problem: Identify the following molecule using IUPAC rules.
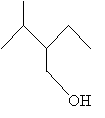
Solution: Find and number the longest carbon chain such that the alcohol group is included and is located at the lowest position number. Also, be sure to maximize the number of substitutive alkyl groups. Then name the molecule according to IUPAC rules.

Alkenes: Nomenclature
An alkene is a hydrocarbon characterized by a double bond between two constituent carbon atoms. Obviously, the name alkene is very close to alkane, and for good reason. Consider, for instance, ethane (an alkane having two carbon atoms) versus ethene (an alkene having two carbon atoms):
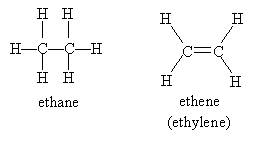
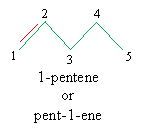
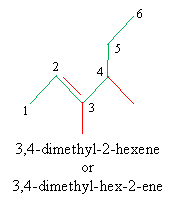
Note that alkenes are named by replacing the -ane ending of an alkane with -ene. The position of the double bond is not ambiguous in the case of ethene (commonly called ethylene) and propene (commonly called propylene), but for longer carbon chains, the position of the double bond is important. To name an alkene according to IUPAC rules, find the longest carbon chain that includes the double bond, number it so that the positions of the carbons containing the double bond are minimized, then write the name following the usual pattern. When numbering the double bond, use the position number for the first carbon (i.e., the lower of the two numbers) in the double bond. Thus, for instance, we would name the molecule below as follows:
Practice Problem: Identify the following molecule.
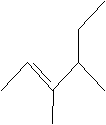
Solution: First, identify the longest carbon chain that includes the double bond, then number the chain appropriately, making note of the substituent alkyl groups. Write the name of the molecule.
Alkynes: Nomenclature
Alkynes are similar to alkenes, except they involve a triple bond between two carbons rather than a double bond. For alkyne nomenclature, the -ene (or -ane) suffix is replaced by -yne. The simplest alkyne, C2H2, goes by the name ethyne or its common name, acetylene. The same rules of alkene nomenclature apply to alkynes. If a double bond and triple bond both appear in the same molecule, the two are treated equally for the purpose of numbering the longest carbon chain. Below are two illustrative examples of alkyne nomenclature. Note in the molecule on the right, the double bond wins in the case of a tie for the numbering scheme.
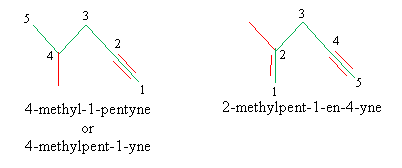
Practice Problem: Identify the following molecule.
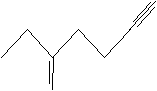
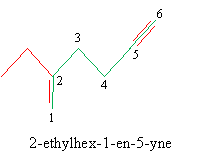
Solution: Number the longest carbon chain containing both multiple bonds, in this case giving preference to the double bond. Write the correct IUPAC name.
For carbon rings, the rules for double bonds (cycloalkenes) and triple bonds (cycloalkynes) are parallel with those for cycloalkanes.



















