Synthesis and Reactions of ?-Bicarbonyl Compounds
Key Terms
- ?-bicarbonyl compound
- Ester
- Acyl group
- Acylation
Objectives
- Identify the characteristics of ?-bicarbonyl compounds (?-diketones)
- Review a synthesis reaction for ?-diketones
- Characterize another representative reaction of ?-diketones
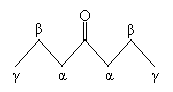
Combining these concepts for now, we will consider the chemical properties of ?-bicarbonyl compounds (also called ?-diketones, 1,3-diketones, and 1,3-bicarbonyl compounds), which involve two carbonyl groups separated by a single carbon atom (whether they are substituted or not). The example of 2,4-heptanedione is depicted below.
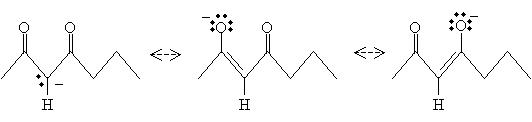
Recall that the ? hydrogen of a carbonyl compound is relatively acidic because of the resonance forms that result in the resulting ion. The same applies in the case of ?-diketones, except it is especially the case for the hydrogens bound to the carbon between the two carbonyl groups. For instance, 2,4-heptanedione is more acidic than acetone, for instance. The resulting conjugate base has three resonance forms, increasing its stability.
Synthesis
Here, we will consider one type of synthesis reaction that yields a ?-diketone: acylation of ketones with esters. But first, we must define some terms. An ester is a compound of the following form.
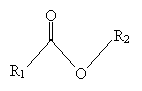
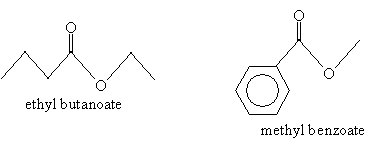
Nomenclature for esters follows the form alkyl alkanoate, where R2 is the alkyl group and R1 (along with the carbonyl and oxygen atom) is the alkanoate group. (Note that the alkanoate group name includes the carbon in the carbonyl group.) Thus, for instance, the molecules below are named as follows.
Practice Problem: Determine the name of the molecule below.
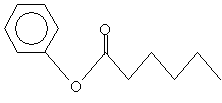
Solution: Notice that the alkyl group is on the left: it is a phenyl group. The alkanoate group in this case is hexaneoate. Thus, the molecule is pheny alkanoate.
An acyl group has the form shown below. Note that it is just another way of naming a major portion of a ketone or aldehyde.
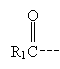
Acylation, as the name indicates, involves addition of an acyl group. One way to form a ?-diketone is through acylation of a ketone using an ester. The ester must not have a corresponding enol form: consider, for instance, methyl benzoate.
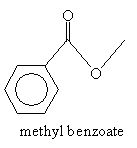
Note that because the molecule has no ? hydrogens, it is not enolizable (it has no corresponding enol form). By adding acetone in the presence of sodium methoxide and an acid, a ?-diketone can be formed by way of the reaction mechanism shown below. First, acetone forms an enolate ion by way of interaction with an methoxide ion.
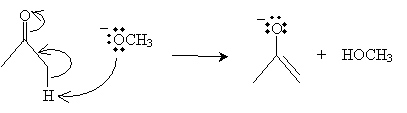
This enolate ion (a nucleophile) can then attack the carbonyl group of the methyl benzoate molecule.
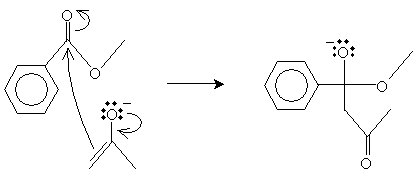
The final step involves elimination of a methoxide ion, leaving a ?-diketone.
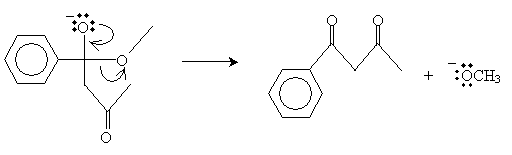
In this case, the product is 1-phenyl-1,2-butanedione.
A similar reaction can occur in a single non-cyclic molecule of the proper arrangement. The result is a cyclic ?-diketone. Consider the molecule below.
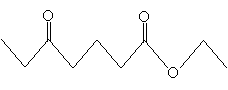
The carbonyl group (on the left side) can form an enolate io, similarly to the mechanism above.
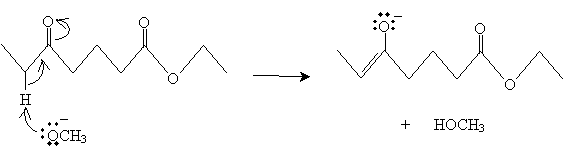
This molecule can then form a ring following the same mechanism as above for two separate molecules.
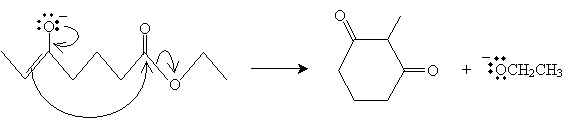
In this case, the product is 2-methyl-1,3-cyclohexanedione. This type of reaction can take place when the product is a five- or six-carbon ring.
Reactions
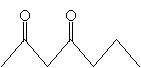
As with synthesis of ?-diketones, we will only consider one reaction of these types of molecules. ?-diketones can undergo alkylation by an alkyl halide in the presences of a weak base. Consider, for instance, 3,5-heptanedione and 1-chloropropane. The overall alkylation reaction is given below.
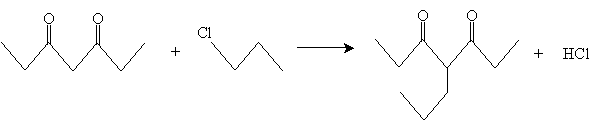
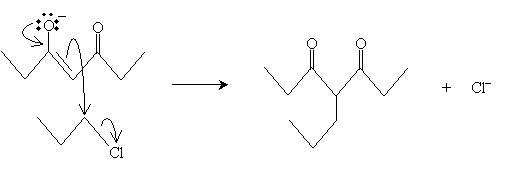
The reaction starts with conversion of the ?-diketone into an enolate ion by way of a base, which abstracts a proton from the carbon between the carbonyl groups.

The enolate ion then acts as a nucleophile in an SN2 reaction with the alkyl halide (1-chloropropane).
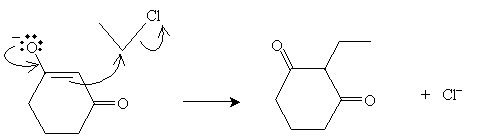
Practice Problem: 1,3-cyclohexanedione is combined with bromoethane. What products result from the ensuing reaction in a weakly basic environment?
Solution: The reactants are depicted below.
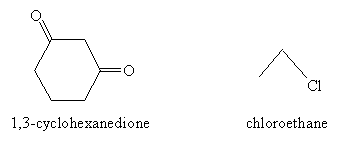
The alkylation reaction first involves conversion of 1,3-cyclohexanedione to its enolate ion form. This ion then reacts as a nucleophile with chloroethane, yielding the product 2-ethyl-1,3-cyclohexanedione.