Introduction Because lipids include a variety of complex molecules that are involved in numerous biological processes, this discussion only lightly introduces these compounds.
Key Terms
- Lipid
- Fat
- Oil
- Fatty acid
- Trans fatty acid
- Trans fat
- Wax
Objectives
- Introduce the general characteristics of lipids and a few specific examples
Types of Lipids
One type of lipid that you are probably familiar with are fats. Fats, like carbohydrates, are energy storage chemicals for organisms, but fats are more efficient per unit mass in storing energy. Oils, another lipid, are differentiated from fats only by way of their physical state: oils are liquids, fats are solids at room temperature. Generally, however, both are referred to as fats.
Fats are mixtures of various triglycerols, the general form of which is shown below.
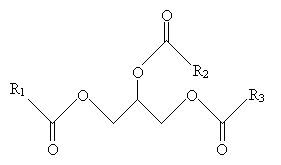
The different triglycerols vary in the composition of the groups R1, R2, and R3, but these groups are generally unbranched carbon chains, possibly with double bonds, and usually have an even number of carbon atoms (usually between 12 and 20).
Another type of lipid, a fatty acid, results from the hydrolysis of a fat. Glycerol is shown below.
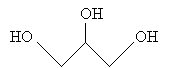
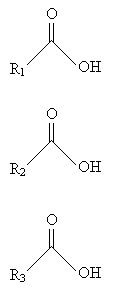
The resulting fatty acids are the following. Notice that these fatty acids are simply carboxylic acids.
An example of a fatty acid is stearic acid (also called octadecanoic acid according to IUPAC systematic nomenclature), shown below.
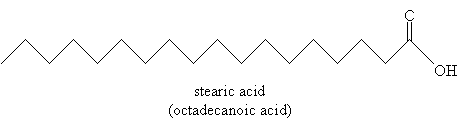
Stearic acid is an example of a saturated fatty acid (recall that a saturated hydrocarbon has no double or triple bonds-it is "saturated with hydrogen). Some fatty acids, however, are unsaturated: oleic acid is one example.
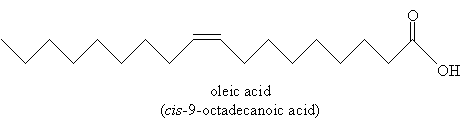
Fatty acids can have multiple double bonds as well. Most unsaturated fatty acids have cis double bonds; some (primarily created artificially) have trans double bonds. These trans fatty acids are increasingly believed to be harmful to humans and other organisms. The process of hydrogenation of a fat converts cis double bonds to trans double bonds, yielding a trans fat.
Another type of lipid is a wax. Waxes are composed of a number of esters (such as that shown below) with long (12 or more carbons), unbranched carbon chains (R1 and R2 below).
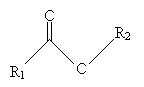
An example of an ester component of a wax is shown below.
Fats, fatty acids, and waxes are just a few types of lipids. Far from covering all the different types of lipids and reactions that involve them, this article provides just an overview.
Practice Problem: The following fat undergoes hydrolysis. What are the resulting products?
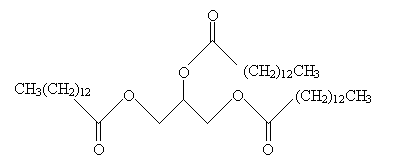
Solution: Hydrolysis of a fat yields glycerol and three fatty acids. Note in this case that the fatty acid constituents of the fat above are all identical. Thus, only two products are yielded in this case. These products are shown below.
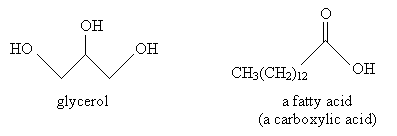
Conclusion
Biochemistry, which is the study of chemical reactions and characteristics in biological systems (organisms), includes other organic compounds such as proteins, amino acids, and nucleic acids. Much in this area is little understood: organisms are still a mystery in many of their chemical functions. Biochemists understand some processes better than others.