We will focus on addition reactions in alkenes and alkynes.
Key Terms
- Unsaturated hydrocarbon
- Saturated hydrocarbon
- Hydrogenation
- Lindlar catalyst
- Electrophile
- Electrophilic addition
- Markovnikov's rule
- Hydration
- Hydrolysis
- Bromination
- Halogenation
Objectives
- Identify addition reactions and their conditions for alkenes and alkynes
Addition Reactions of Alkenes and Alkynes
Addition reactions are, essentially, the opposite of elimination reactions. Recall that an elimination reaction had the following general form:
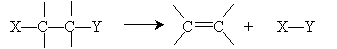
An addition reaction is simply the reverse of this process:
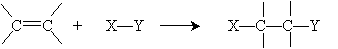
Clearly, the same substances are present in both cases, so the difference between the reaction that prefers one side (say, the products) over the other side (the reactants) is the conditions under which the reaction occurs: other chemicals that may be present, temperature, and so on.
For alkynes, the general elimination reaction is shown below, followed by the corresponding addition reaction.
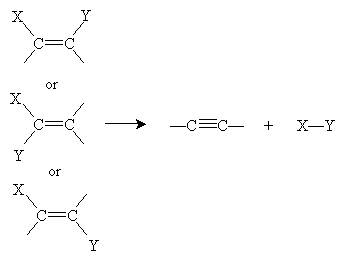
Hydrogenation
Alkenes and alkynes are called unsaturated hydrocarbons because, as the name indicates, the carbon atoms are not "saturated" with hydrogens, owing to the presence of double or triple carbon-carbon bonds. As a result, these substances tend to be more reactive at the site of the multiple bond than a saturated hydrocarbon (an alkane or cycloalkane), which cannot accept any additional atoms. In the presence of heat, saturated hydrocarbons can eliminate hydrogen gas to form unsaturated hydrocarbons (alkenes and alkynes). The reverse process, hydrogenation, can be performed in the presence of a catalyst like metallic platinum. Hydrogen gas reacts with the metal surface, breaking the hydrogen-hydrogen bond to form weaker metal-hydrogen bonds. An alkene or alkyne can then react with the metal in a similar manner, then form stronger bonds with two or more hydrogen atoms. This process is illustrated below for a generic alkene.
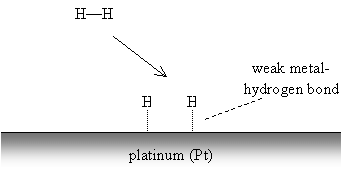
The alkene forms similar weak carbon-metal bonds at the site of the double bond.
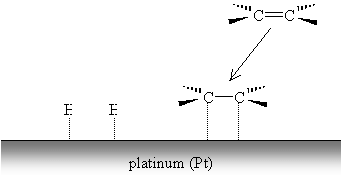
The weak metal bonds are then replaced by stronger carbon-hydrogen bonds, and the alkene (or alkyne, by a similar process) becomes saturated.
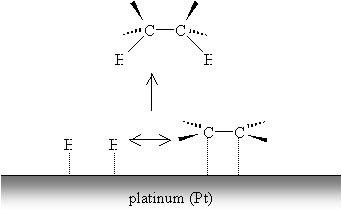
The process of hydrogenation does not occur such that both carbons are saturated simultaneously. Nevertheless, hydrogenation, as the diagram indicates, results in addition of hydrogen atoms to the same "side" of the alkene. (This result is more apparent in the case of cycloalkenes, however.) In the case of alkynes, the hydrogenation process can produce alkenes (rather than completely saturated alkanes) through the use of a so-called Lindlar catalyst, like Lindlar palladium.
Practice Problem: The following molecule is combined with hydrogen gas in the presence of Lindlar palladium. What is the product of the reaction?

Solution: A Lindlar catalyst only hydrogenates alkynes. Thus, the product molecule is a diene (hydrocarbon with two double bonds), which is shown below. The IUPAC name is 7-methyl-1,6-octadiene.

Electrophilic Addition
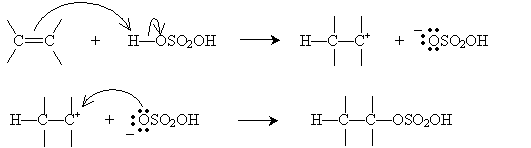
As the name implies, an electrophile is an "electron-loving" or "electron-seeking" compound that can act as a Lewis acid (electron pair acceptor). Electrophiles can react with the double bond of an alkene, resulting in an electrophilic addition reaction. Consider the simple case of ethylene (ethene) reacting with a hydrogen halide, HX (where X is the halogen). Hydrogen halides are dipolar molecules, and the proton side can approach the 2p orbital of ethylene and accept an electron pair (i.e., act as a Lewis base). The ensuing reaction is shown below. First, the rate-determining step involves creation of a halide ion and a carbocation.
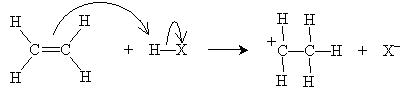
The carbocation, which readily accepts an electron pair, then reacts with the halide ion to form an alkyl halide.
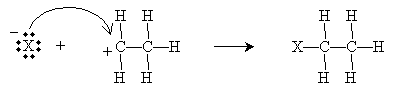
Overall, then, the reaction is summarized below.
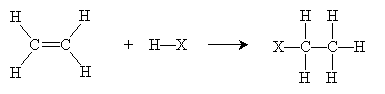
In the case of addition to more complicated alkenes, Markovnikov's rule states that the proton adds to the carbon with the most hydrogen atoms attached, and the halogen adds to the carbon with the fewest hydrogen atoms attached. Thus, for instance, addition of HCl to 2-methyl-2-pentene yields 2-methyl-2-chloropentane.
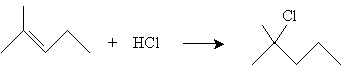
Similarly, alkenes can be converted into alcohols by a reverse process involving concentrated sulfuric acid. This process is called hydration (as opposed to dehydration, which converts an alcohol into an alkene). To begin, sulfuric acid adds to certain alkenes as follows.
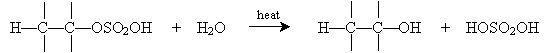
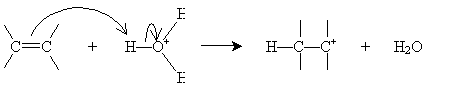
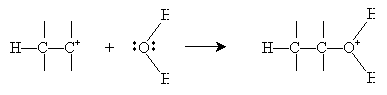
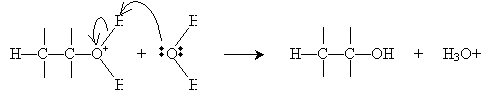
Alternatively, hydration can be performed in a more dilute sulfuric acid solution. The alkene reacts with hydronium (H3O+) to form a carbocation that then reacts with water.
Halogens can also be added to alkenes in the presence of, for instance, methyl chlorides like tetrachloromethane (carbon tetrachloride, CCl4), chloroform (trichloromethane, CHCl3), and dichloromethane (CH2Cl2). The example reaction below illustrates the mechanism for addition of bromine to hexene (also called bromination, or more broadly, halogenation). Note that because of the mechanics of this reaction, halogen atoms add to opposite "sides" of the double bond.
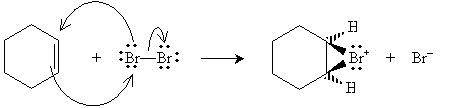
The bromide ion must then approach from the "bottom" (into the surface of the page) of the hexane molecule.
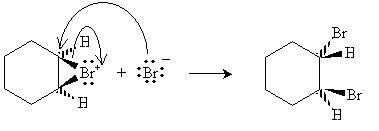
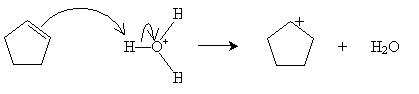
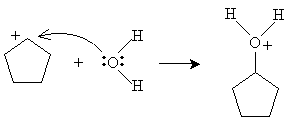
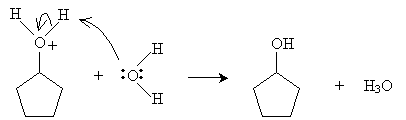
Practice Problem: Cyclopentene is added to a dilute solution of sulfuric acid. What product is yielded?
Solution: Cyclopentene reacts with hydronium ions to form a carbocation that reacts with water to produce an alcohol, as shown below. The product is cyclopentanol.
Addition reactions to alkynes are similar to additions to alkenes. For instance, hydrogen halides can be added to an alkyne by way of a mechanism similar to that of alkenes. Consider the addition of HX (a generic hydrogen halide) to 1-propyne; according to Markovnikov's rule, the halogen adds to the carbon with the fewest hydrogen atoms.
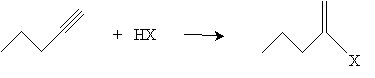
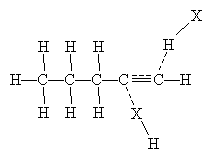
The mechanism for this reaction seems to be a cross between a single-step addition involving three molecules and a two-step addition involving a carbocation intermediate molecule. Two hydrogen halide molecules approach the triple bond and, owing to the dipole moment of the hydrogen halides, form weak bonds represented by dashed lines in the diagram below.
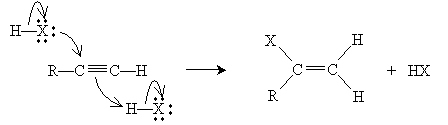
The overall mechanism for the addition is shown below. The propyl group attached to the leftmost carbon of the triple bond shown above is represented as R for simplicity.
Thus, when in the presence of hydrogen chloride, for instance, 1-pentyne forms 2-chloro-1-pentene. If large amounts of the hydrogen halide are present, another addition reaction can take place following the mechanism for alkenes. The result, using our example of 1-pentyne in hydrogen chloride, would be 2,2-dichloropentane.
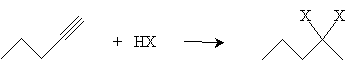
Alkynes can also be halogenated by adding chlorine or bromine. When present in equal amounts, the halogen atoms add in a trans manner and yield a dihaloalkene, such as that shown below for 3-hexyne.
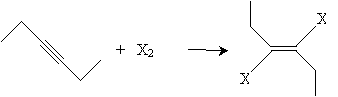
If the halogen is present in concentrated amounts, it may also add to the double bond.
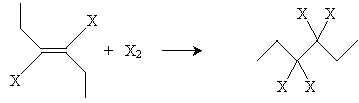
The final result in this case (for chlorine) is 3,3,4,4-tetrachlorohexane.
Alkynes can also undergo hydration.
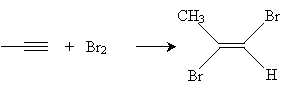
Practice Problem: Propyne is added to a dilute solution of bromine. What is the product of the reaction?
Solution: Propyne is shown below. In a dilute bromine solution, the bromine adds to the alkyne to produce an alkene (propylene, in this case) with two halogen atoms. The result is trans-1,2-dibromopropene. Because the solution is dilute, the reaction does not progress to add more bromine atoms to the alkene.